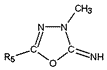
"Zur Kenntnis der 2-Imino-1,3,4-oxadiazoline", Tabelle 8, Manfred Just, Dissertation B, 1976, Universität Potsdam

"Zur Kenntnis der 2-Imino-1,3,4-oxadiazoline",
Tabelle 8,
Manfred Just, Dissertation B, 1976, Universität Potsdam
Nr. |
R5 |
Säure |
Summenformel |
Schmelzp. |
Ausb. |
Analyse (ber.:/gef.:) |
||
1 |
Wasserstoff |
CH3OSO3H |
C4H9N3O5S |
Öl |
64 c) |
22,75 |
4,30 |
19,90 |
2 |
Wasserstoff |
- |
C3H5N3O |
Öl |
25 d) |
36,36 |
5,09 |
42,40 |
3 |
Methyl |
CH3OSO3H |
C5H11N3O5S |
78 - 91 |
60 c) |
26,66 |
4,92 |
16,66 |
4 |
Benzyl |
CH3OSO3H |
C11H15N3O5S |
110 - 167 Z |
68 c) |
44,14 |
5,05 |
14,04 |
5 |
Phenyl |
CH3OSO3H |
C10H13N3O5S |
160 - 170 |
99 c) |
- |
- |
14,63 |
6 |
Phenyl |
HCl |
C9H10ClN3O |
211 - 223 |
62 a) |
51,08 |
4,76 |
19,65 |
7 |
Phenyl |
- |
C9H9N3O |
102 - 104 |
97 d) |
61,70 |
5,18 |
23,99 |
8 |
p-Tolyl | - | C10H11N3O 189,22 |
107-109 | - |
63,48 63,21 |
5,86 6,34 |
- |
9 |
o-Chlorphenyl |
- |
C9H8C1N3O |
71 |
- |
51,57 |
3,85 |
- |
10 |
m-Chlorphenyl |
CH3OSO3H |
C10H12ClN3O5S |
125 - 132 |
93 c) |
- |
- |
13,06 |
11 |
m-Chlorphenyl |
- |
C9H8C1N3O |
100 - 103 |
91 d) |
51,57 |
3,85 |
20,04 |
12 |
p-Chlorphenyl |
CH3OSO3H |
C10H12C1N3O5S |
210 - 214 Z |
99 c) |
- |
- |
13,06 |
13 |
p-Chlorphenyl |
- |
C9H8C1N3O |
112 - 113 |
90 d) |
51,57 |
3,85 |
20,04 |
14 |
o-Bromphenyl | - | C9H8BrN3O 254,09 |
64-66 | 62 e) |
42,54 42,44 |
3,17 3,28 |
- |
15 |
m-Bromphenyl | - | C9H8BrN3O 254,09 |
112-114 | 64 e) |
42,54 42,50 |
3,17 3,42 |
- |
16 |
p-Bromphenyl | - | C9H8BrN3O 254,09 |
136-137 | 48 e) |
42,54 42,59 |
3,17 3,05 |
- |
17 |
m-Iodphenyl | - | C9H8IN3O 301,09 |
109-111 | 62 e) |
35,90 35,48 |
2,68 2,91 |
- |
18 |
p-Iodphenyl | - | C9H8IN3O 301,09 |
116-118 | 64 e) |
35,90 35,98 |
2,68 3,15 |
- |
19 |
o-Nitrophenyl |
CH3OSO3H |
C10H12N4O7S |
ab 120 Z |
90 c) |
- |
- |
16,86 |
20 |
o-Nitrophenyl |
HCl |
C9H9ClN4O3 |
190 - 192 |
87 a) |
- |
- |
21,83 |
21 |
o-Nitrophenyl |
- |
C9H8N4O3 |
81 - 83 |
98 d) |
49,09 |
3,66 |
25,44 |
22 |
m-Nitrophenyl |
CH3OSO3H |
C10H12N4O7S |
153 - 160 Z |
96 c) |
- |
- |
16,86 |
23 |
m-Nitrophenyl |
HC1 |
C9H9ClN4O3 |
205 - 207 Z |
61 c) |
- |
- |
21,83 |
24 |
m-Nitrophenyl |
- |
C9H8N4O3 |
122 - 123 |
75d) |
49,09 |
3,66 |
25,44 |
25 |
p-Nitrophenyl |
CH3OSO3H |
C10H12N4O7S |
221 - 223 Z |
98 c) |
- |
- |
16,86 |
26 |
p-Nitrophenyl |
HC1 |
C9H9C1N4O3 |
189 - 197 Z |
95 a) |
- |
- |
21,83 |
27 |
p-Nitrophenyl |
- |
C9H8N4O3 |
255 - 256 |
91 d) |
49,09 |
3,66 |
25,44 |
28 |
o-Methoxyphenyl |
CH3OSO3H |
C11H15N3O5S |
132 - 140 |
35 c) |
- |
- |
13,24 |
29 |
o-Methoxyphenyl |
HC1 |
C10H12C1N3O2 |
193 - 195 |
42 a) |
- |
- |
17,39 |
30 |
o-Methoxyphenyl |
- |
C10H11N3O2 |
92 - 94 |
71 d) |
58,53 |
5,40 |
20,48 |
31 |
p-Methoxyphenyl |
CH3OSO3H |
C11H15N3O6S |
175 - 179 |
92 c) |
- |
- |
13,24 |
32 |
p-Methoxyphenyl |
- |
C10H11N3O2 |
142 - 143 |
97 d) |
58,53 |
5,40 5,46 |
20,48 |
33 |
Naphthyl-(l) |
CH3OSO3H |
C14H14N3O5S |
188 - 194 Z |
97 c) |
- |
- |
12,49 |
34 |
Naphthyl-(1) |
- |
C13H11N3O |
137 - 139 |
83 d) |
69,32 69,17 |
4,92 |
18,65 |
35 |
Naphthyl-(2) |
CH3OSO3H |
C14H14N3O5S |
203 - 207 Z |
94 c) |
- |
- |
12,49 |
36 |
Naphthyl-(2) |
- |
C13H11N3O |
142 - 143 |
99 d) |
69,32 |
4,92 |
18,65 |
37 |
Furyl-(2) |
CH3OSO3H |
C8H11N3O6S |
135 - 139 |
57 c) |
34,66 |
4,00 4,56 |
15,16 |
38 |
Thienyl-(2) |
CH3OSO3H |
C8H11N3O5S2 |
125 - 135 Z |
87 c) |
32,76 |
3,78 |
14,33 |
a) bezogen auf das N-Cyan-N-methyl-hydrazin
c) bezogen auf das eingesetzte 2-Amino-l ,3,4-oxadiazol bei der Reaktion mit Methylierungs-Mitteln
d) bezogen auf entsprechende eingesetzte Amino-oxadiazolin-Salz
e) "Zur Kenntnis der 2-Imino-1,3,4-oxadiazoline", Sauer, Erika; Dissertation (1980), Universität Erfurt
Z = Zersetzung
Für die Ausbeute der Methylierung von Amino-oxadiazolen ist die Art des Methylierungsmittels von Bedeutung.
Tabelle 6
Methylierung des 2-Amino-5-phenyl-1,3,4-oxdiazols
Methylierungsmittel |
Reaktionsbedingung |
Ausbeute an |
Dimethylsulfat |
30 min, 140° C |
99 % |
Chlorsulfonsäuremethylester |
30 min, 140° C |
54 % |
p-Toluolsulfonsäuremethylester |
30 min, 140° C |
36 % |
Methyljodid |
10 h, Rückfluss |
28 % |
Methyljodid |
30 min, 140° (Rohr) |
38 % |
Dimethylsulfit |
4 h, Rückfluss |
- |