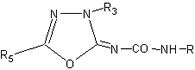
"Zur Kenntnis der 2-Imino-1,3,4-oxadiazoline", Tabelle 39
Manfred Just, Dissertation B, 1976, Universität Potsdam
| Nr. |
R |
R3 |
R5 |
Summenfor. |
Schmelzp. |
Ausb. |
ber.:gef: % N |
IR-Absorption |
||
| 1 |
Methyl |
Methyl |
Phenyl |
C11H12N4O2 232,24 |
223-225 |
83 a) |
24,12 |
1565 |
1630 |
1690 |
| 2 |
Methyl |
Phenyl |
Phenyl |
C16H14N4O2 294,32 |
114-115 | 79 a) |
19,04 |
1530 |
1665 |
1700 |
| 3 |
Phenyl |
Phenyl |
Phenyl |
C21H16N4O2 356,39 |
186-183 |
96 a) |
15,72 15,69 |
1530 |
1660 |
1635 |
| 4 |
Phenyl |
Äthyl |
p-Nitro- |
C17H15N5O4 353,34 |
216-218 |
91 a) |
19,82 19,63 |
1535 |
1650 |
1690 |
| 5 |
Acetyl |
Phenyl |
Phenyl |
C17H14N4O3 322,33 |
213-219 |
87 a) |
17,38 17,57 |
1530 |
1645 1665 |
1695 |
| 6 |
Acetyl |
Phenyl |
o-Chlor- |
C17H13ClN4O3 356,77 |
182-184 |
85 a) |
15,70 |
1520 |
1645 |
1695 |
| 7 |
Acetyl |
Phenyl |
o-Nitro- |
C17H13N5O5 367,32 |
191-193 |
92 a) |
19,07 18,89 |
1515 |
1640 1658 |
1690 |
| 8 |
Acetyl |
Phenyl |
m-Nitro- |
C17H13N5O |
196-198 |
83 a) |
19,07 18,91 |
1515 |
1635 1655 |
1690 |
| 9 |
Acetyl |
Phenyl |
p-Nitro- |
C17H13N5O |
236-238 |
94 a) |
19,07 18,79 |
1530 |
1630 1655 |
1685 |
a) bezogen auf Imino-oxadiazolin